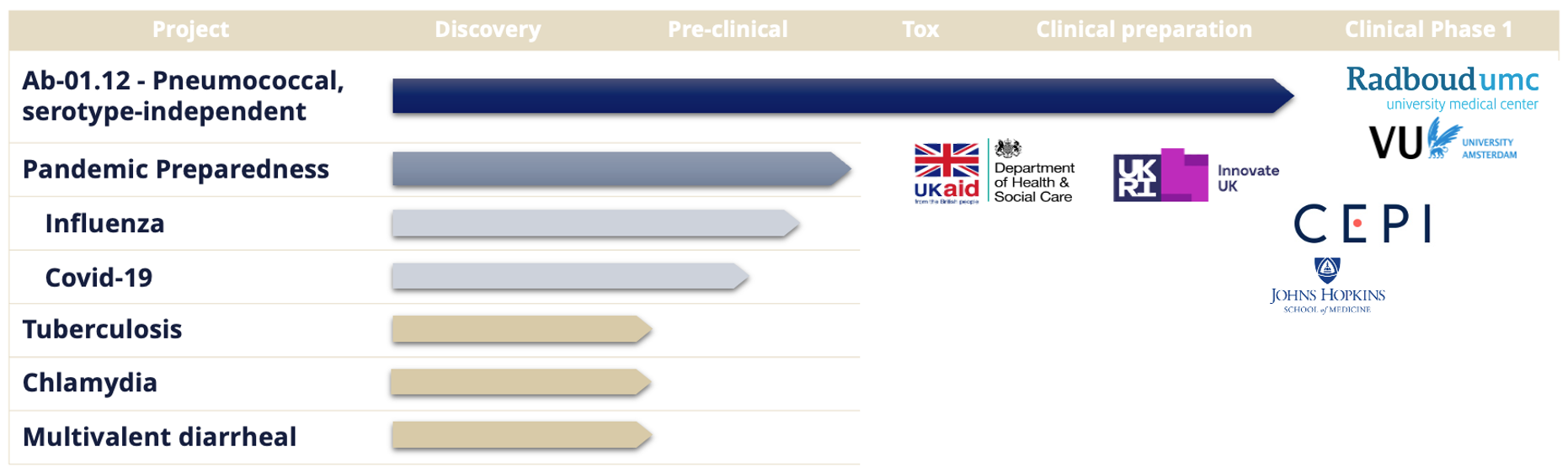
Seasonal and pandemic influenza continue to pose major global health challenges, with millions of infections each year and periodic emergence of highly transmissible or virulent strains. Despite widespread use of current vaccines, effectiveness varies significantly between seasons, largely due to rapid viral mutation and the need to predict circulating strains months in advance. Abera’s intranasal influenza vaccine candidate is designed to address these limitations by presenting HA antigens at high density on OMVs, generating strong mucosal immunity at the site of infection while remaining adaptable to new variants as they arise. Preclinical studies show robust and durable IgA and IgG responses, supporting the potential for both improved protection and reduced transmission. The program is in advanced preclinical development and continues to demonstrate the platform’s capacity for rapid antigen updating in both seasonal and pandemic scenarios.